Subcellular antioxidant defense via redox-reactive fluorescent sensor
Project Summary
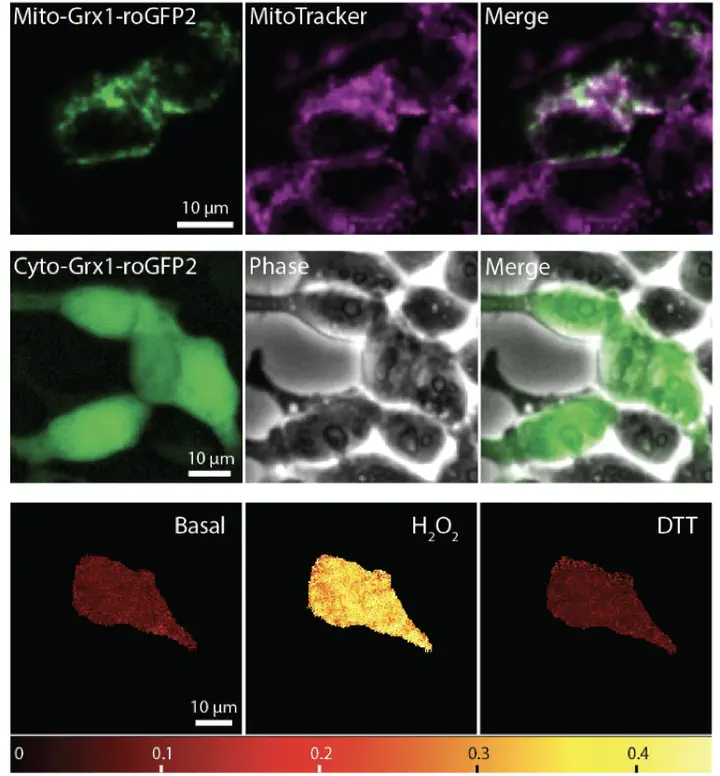
In this work, we reported a new method using combined approaches of organelle-specific redox sensor Grx1-roGFP2 and non-targeted proteomics to investigate the real-time Cu-dependent antioxidant defenses of mitochondria and cytosol in live HEK293 cells. The time-dependent subcellular redox results revealed that subcellular Cu-induced oxidative stress happens with much slower rates and fewer extents than H2O2. Mitochondria appear to have more efficient restoration of Cu-dependent GSH/GSSG ratio than the cytosol. The corresponding proteomics data show that several mitochondrial proteins involved in mitochondrial oxidative phosphorylation and GSH synthesis rapidly undergo significant abundance changes after prolonged Cu treatments. In contrast, cytosolic proteins involved in GSH synthesis undergo substantial changes after Cu reduction. In other words, mitochondria and cytosol have different antioxidant defense mechanisms upon Cu-dependent oxidative stress despite both modulating GSH/GSSG system. Mitochondria respond to Cu treatments and modify protein profiles more significantly than the cytosol. These findings provide information on how redox status changes within cellular compartments and triggers organelle-specific protein abundance changes in response to Cu-level changes, highlighting the interplay mechanisms between redox and Cu homeostasis.